SEATTLE, Nov. 19, 2013 /PRNewswire/ -- Omeros Corporation (NASDAQ: OMER) today reported analyses of Phase 3 clinical data showing the impact of OMS302 on reducing the incidence of miosis (pupil constriction) during intraocular lens replacement (ILR). OMS302 is the company's proprietary Pharmacosurgery® product being developed for all ILR, including cataract surgery and refractive lens exchange.
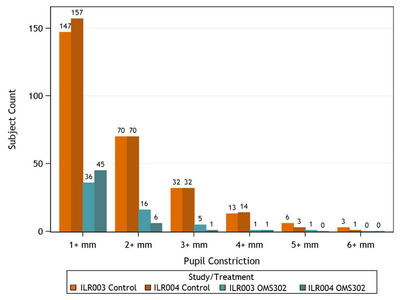
The analyses were presented by Steve Whitaker, M.D., Omeros' vice president of clinical development and chief medical officer on November 17 at the Annual American Academy of Ophthalmology (AAO) Meeting in New Orleans. The clinical data presented at the AAO meeting and shown below demonstrated that, in these clinical trials in which all patients received standard-of-care preoperative mydriatic topical drops, OMS302 reduced the occurrence of intraoperative pupil constriction. Intraoperative miosis is an important risk factor for surgical complications. As shown in the figure below, pupil constriction of at least 3 millimeters was common in control patients while constriction greater than 1 millimeter was uncommon in OMS302-treated patients.
(Photo: http://photos.prnewswire.com/prnh/20131119/MM19781 )
"We are pleased with the recognition that the recent clinical data for OMS302 received from the ophthalmic community during the AAO annual meetings," said Gregory A. Demopulos, M.D., chairman and chief executive officer of Omeros. "Ophthalmologists continue to appreciate the potential clinical benefits of OMS302, and we look forward to the drug's expected commercial launch in 2014."
About Omeros' OMS302 Program
OMS302 is Omeros' product being developed for use during intraocular lens replacement (ILR), including cataract surgery and refractive lens exchange. OMS302 is a proprietary combination of the mydriatic (pupil dilating) agent phenylephrine and the anti-inflammatory agent ketorolac. Omeros' New Drug Application (NDA) for OMS302 has been accepted for filing by the FDA and its Marketing Authorization Application (MAA) for OMS302 has been validated by the EMA.
ILR involves replacement of the original lens of the eye with an artificial intraocular lens. These procedures are typically performed to replace a lens opacified by a cataract or to correct a refractive error of the lens (i.e., refractive lens exchange). OMS302 is added to standard irrigation solution used in ILR and delivered within the eye to maintain intraoperative mydriasis (pupil dilation), to reduce surgically induced miosis (pupil constriction), and to reduce postoperative pain and irritation. Maintenance of mydriasis is critical to the safety and surgical ease of the procedure. Intraoperative pupil constriction increases the risk of injury to intraocular structures and can substantially prolong surgical time.
About Omeros Corporation
Omeros is a clinical-stage biopharmaceutical company committed to discovering, developing and commercializing small-molecule and protein therapeutics targeting inflammation, coagulopathies and disorders of the central nervous system. Derived from its proprietary PharmacoSurgery® platform, the Company's lead drug product, OMS302 for lens replacement surgery, is currently under review for marketing approval by both the US Food and Drug Administration and the European Medicines Agency with commercial launch planned for 2014. Omeros' five other clinical programs are focused on schizophrenia, Huntington's disease and cognitive impairment; addictive and compulsive disorders; complement-related diseases; and preventing problems associated with surgical procedures. Omeros also has a proprietary GPCR platform, which is making available an unprecedented number of new GPCR drug targets and corresponding compounds to the pharmaceutical industry for drug development.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, which are subject to the "safe harbor" created by those sections for such statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as "anticipate," "believe," "could," "estimate," "expect," "goal," "intend," "may," "plan," "potential," "predict," "project," "should," "will," "would" and similar expressions. Forward-looking statements are based on management's beliefs and assumptions and on information available to management only as of the date of this press release. Omeros' actual results could differ materially from those anticipated in these forward-looking statements for many reasons, including, without limitation, risks associated with Omeros' unproven preclinical and clinical development activities, regulatory oversight, product commercialization, intellectual property claims and the risks, uncertainties and other factors described under the heading "Risk Factors" in the Company's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 7, 2013. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and the Company assumes no obligation to update these forward-looking statements, even if new information becomes available in the future.
Image with caption: "Data from Omeros' Phase 3 Clinical Program (Studies ILR003 and ILR004): Effect of OMS302 vs Control on Miosis." Image available at: http://photos.prnewswire.com/prnh/20131119/MM19781
SOURCE Omeros Corporation
Jennifer Cook Williams, Cook Williams Communications, Inc., Investor and Media Relations, 360.668.3701, jennifer@cwcomm.org